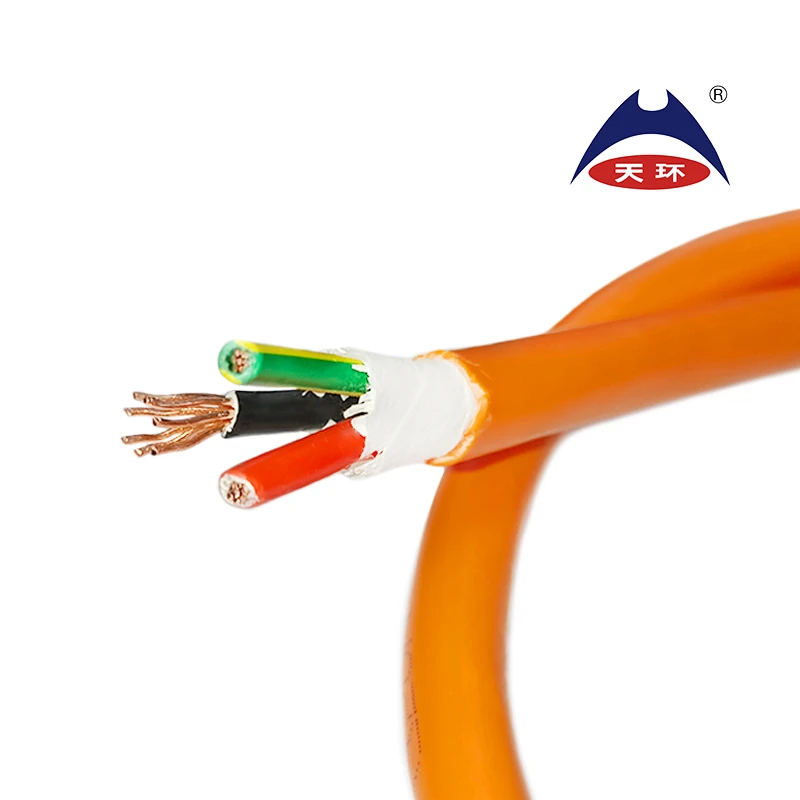
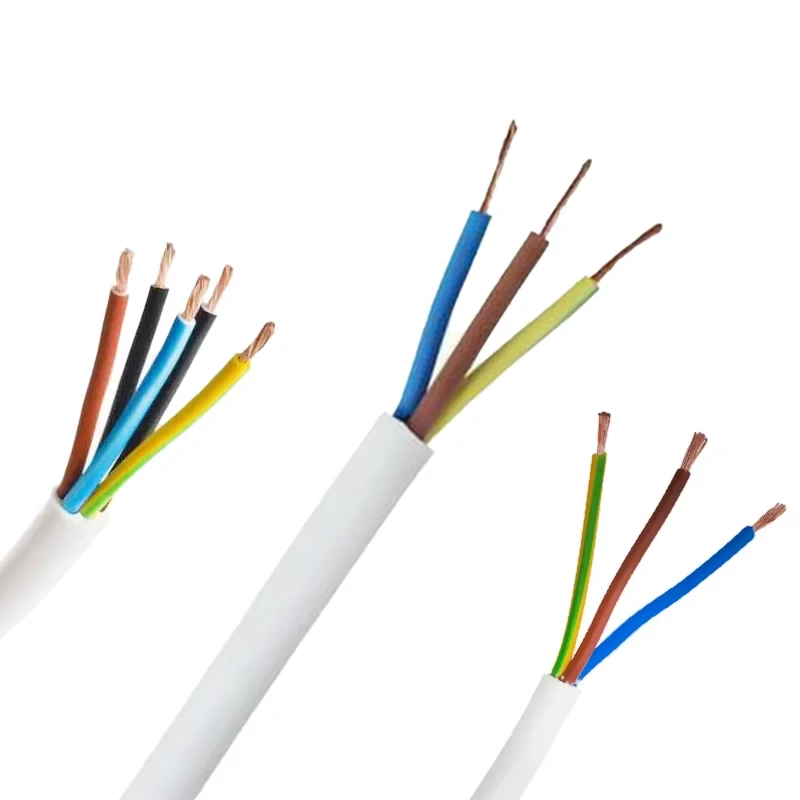
famous iec 60245 3
Understanding IEC 60601-1 The Cornerstone of Medical Electrical Equipment Safety
In the ever-evolving field of healthcare, the importance of safety and performance in medical electrical equipment cannot be overstated. The International Electrotechnical Commission (IEC) has laid down comprehensive standards to ensure the safety and efficacy of these devices, with IEC 60601-1 being one of the most significant. This standard serves as a pivotal framework for manufacturers, regulatory bodies, and healthcare practitioners to ensure that medical equipment is safe for use in various healthcare settings.
A Brief Overview of IEC 60601-1
IEC 60601-1 is a widely recognized international standard that delineates the general requirements for the basic safety and essential performance of medical electrical equipment. The standard was first published in 1977 and has undergone several revisions to address the advances in technology and the associated risks. The latest edition, IEC 60601-12012, incorporates critical updates that reflect modern safety concerns, technological advancements, and the complexities of medical devices used today.
The Core Objectives of IEC 60601-1
The primary objective of IEC 60601-1 is to establish a framework for the safe design and operation of medical electrical equipment. It aims to protect patients, operators, and the environment from hazards that may arise due to electrical devices. This standard sets out requirements addressing various elements, such as electrical shock, electromagnetic compatibility, mechanical hazards, and thermal safety. Additionally, it emphasizes minimizing risks related to the misuse of medical devices, which is crucial for ensuring patient safety.
Key Components of IEC 60601-1
1. General Safety Requirements The standard outlines fundamental safety principles, including protection against electrical shock, hazardous moving parts, and mechanical failure. It mandates that devices must be designed in ways that prevent unintentional operation, reducing the risk of accidents.
famous iec 60245 3
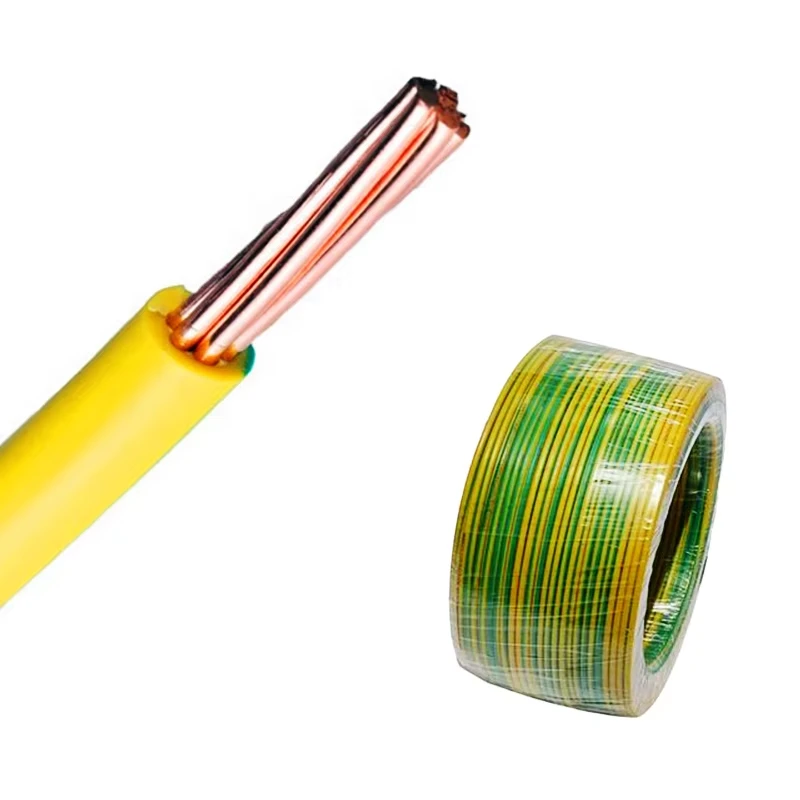
2. Risk Management IEC 60601-1 places significant emphasis on the importance of risk management throughout the product lifecycle. Manufacturers are required to conduct thorough risk assessments and implement control measures to mitigate potential hazards.
3. Essential Performance The standard distinguishes between basic safety and essential performance. Essential performance refers to the performance of a device that is necessary for it to achieve its intended use safely. This component ensures that the equipment not only minimizes risks but also functions effectively.
4. Electromagnetic Compatibility (EMC) In our interconnected world, electromagnetic interference has become a critical concern. IEC 60601-1 includes provisions for EMC to ensure that medical devices operate reliably in the presence of electromagnetic disturbances, thereby safeguarding patient care.
5. Labeling and Instructions for Use Clear labeling and user instructions are vital for the safe operation of medical devices. The standard requires manufacturers to provide comprehensive documentation to inform users about potential risks, proper usage, and maintenance.
The Global Impact of IEC 60601-1
The significance of IEC 60601-1 extends beyond regulatory compliance; it fosters a culture of safety and quality in the healthcare industry. Adherence to this standard not only enhances patient safety but also promotes trust in medical technologies. Regulatory bodies around the world, including the FDA in the United States and the European Medicines Agency in the EU, reference this standard as part of their regulatory processes for approving medical devices.
In conclusion, IEC 60601-1 stands as a foundational element in the realm of medical electrical equipment safety. It aligns manufacturers with best practices, facilitates safe use in clinical environments, and ultimately contributes to improved patient outcomes. As medical technology continues to advance, the relevance of IEC 60601-1 will only grow, ensuring that safety remains a top priority in healthcare innovation. For manufacturers, adherence to this standard is not merely a regulatory necessity but a commitment to the safety and well-being of patients and healthcare providers alike.
-
Reliable LIYCY Cable Solutions for Low and Medium Voltage ApplicationsNewsJul.14,2025
-
Premium Overhead Electrical Wire Solutions for Low and Medium Voltage ApplicationsNewsJul.14,2025
-
Innovative XLPE Electrical Cable Solutions for Modern Low and Medium Voltage NetworksNewsJul.14,2025
-
High-Quality Ethylene Propylene Rubber Cable – Durable EPDM Cable & 1.5 mm 3 Core OptionsNewsJul.14,2025
-
Exploring the Versatility of H1Z2Z2-K 1X4mm2 Cables in Modern ApplicationsNewsJul.14,2025
-
Uses of Construction WiresNewsJul.14,2025
-
Types of Neoprene CableNewsJul.14,2025